Inteprity®

Inteprity®
Inteprity is a first-in-class, animal-use only, in-feed antibiotic for use in prevention of mortality caused by necrotic enteritis (NE) associated with Clostridium perfringens in broiler chickens. Driven by consumer demand, the reduction of antibiotic use and increase in coccidiosis vaccination in poultry has led to a rising incidence of NE. Inteprity lets you battle NE and still meet consumer demand for poultry raised without the use of shared-class antibiotics.
Bag Size
25 kg (55.12 lb)
NA
- Start as Late As Day 18 — Feed for 21 consecutive days to meet the peak NE challenge period.
- Flexible Dose Range — Veterinarians can specify a dose ranging from 13.6 to 40.9 g/ton, based on their assessment of NE risk.
- Animal-Only Antibiotic — This first-in-class product answers growing consumer demand for reduction of shared-class antibiotic use in poultry production.
- Proven By Research — Studies show Inteprity beats leading competitors for controlling NE and promoting average weight gain and feed conversion.1,2
Inclusion Rate: 13.6 to 40.9 grams per ton Type C medicated feed (15-45 ppm) as the sole ration for 21 consecutive days.
Inteprity Protects Your Birds and Bottom Line Against Necrotic Enteritis
With Inteprity, be confident you’re controlling NE in a way that meets customers' demands without compromising bird health and your bottom line.
Inteprity offers a wide dose range, from 13.6 to 40.9 g/ton, to allow veterinarians the flexibility to specify a targeted dose that supports their risk assessment for NE.
The ability to start Inteprity as late as day 18 and feed for 21 consecutive days lets you fight NE during peak challenge.
You can start Inteprity as late as day 18 and use it in combination with Maxiban®, Monteban®, Coban® or Bio-Cox® (salinomycin sodium).
Inteprity’s proven efficacy and flexibility mean you can do what’s right for your birds and still meet customer demand.
Inteprity in the Field
An integrated broiler producer conducted a field trial comparing the effects of Inteprity, BMD® and Stafac® on both mortality caused by NE and performance in 21 million broilers during the summer and fall growing periods.1 Chart 1 shows the results of the two head-to-head comparisons.
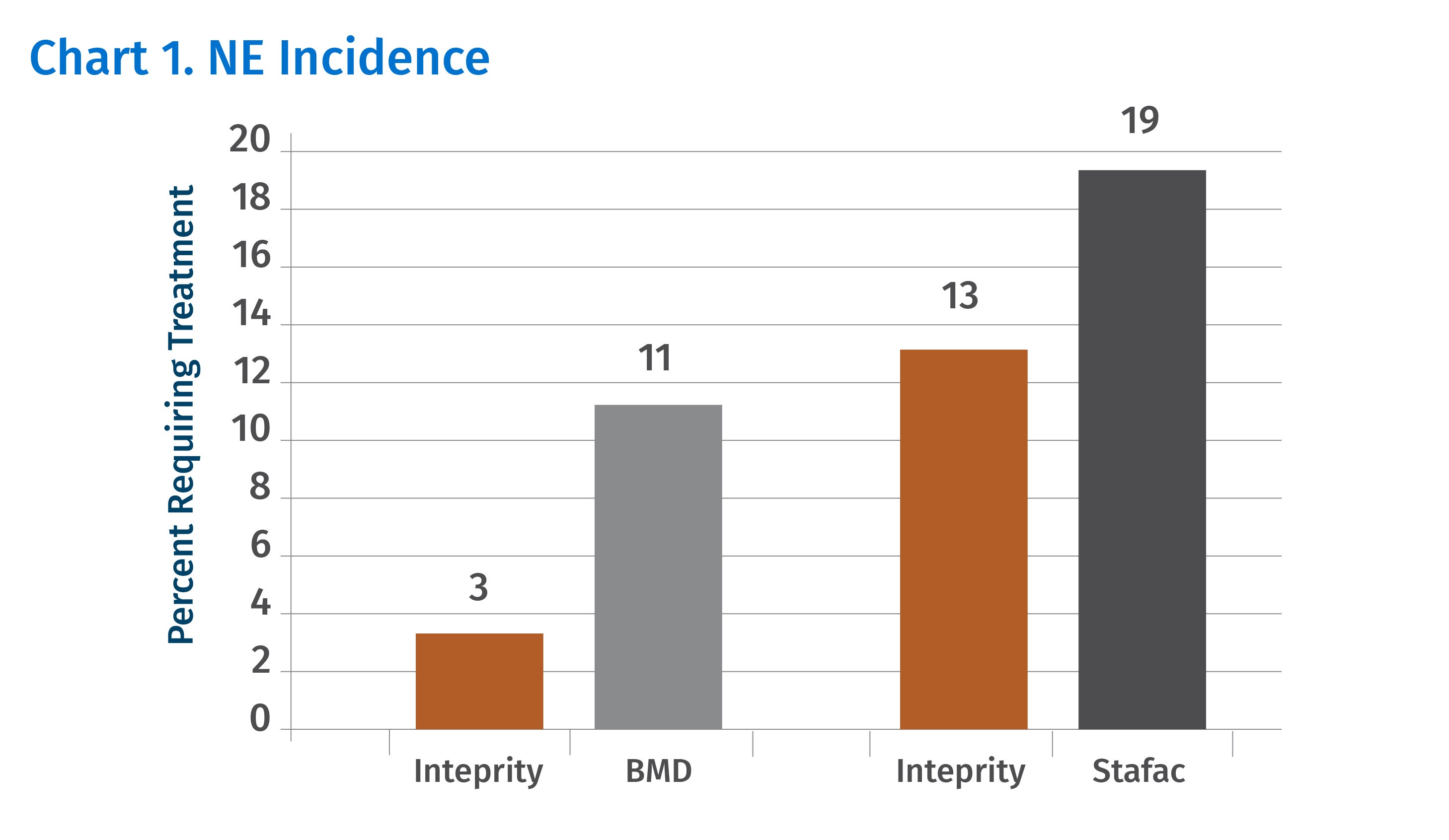
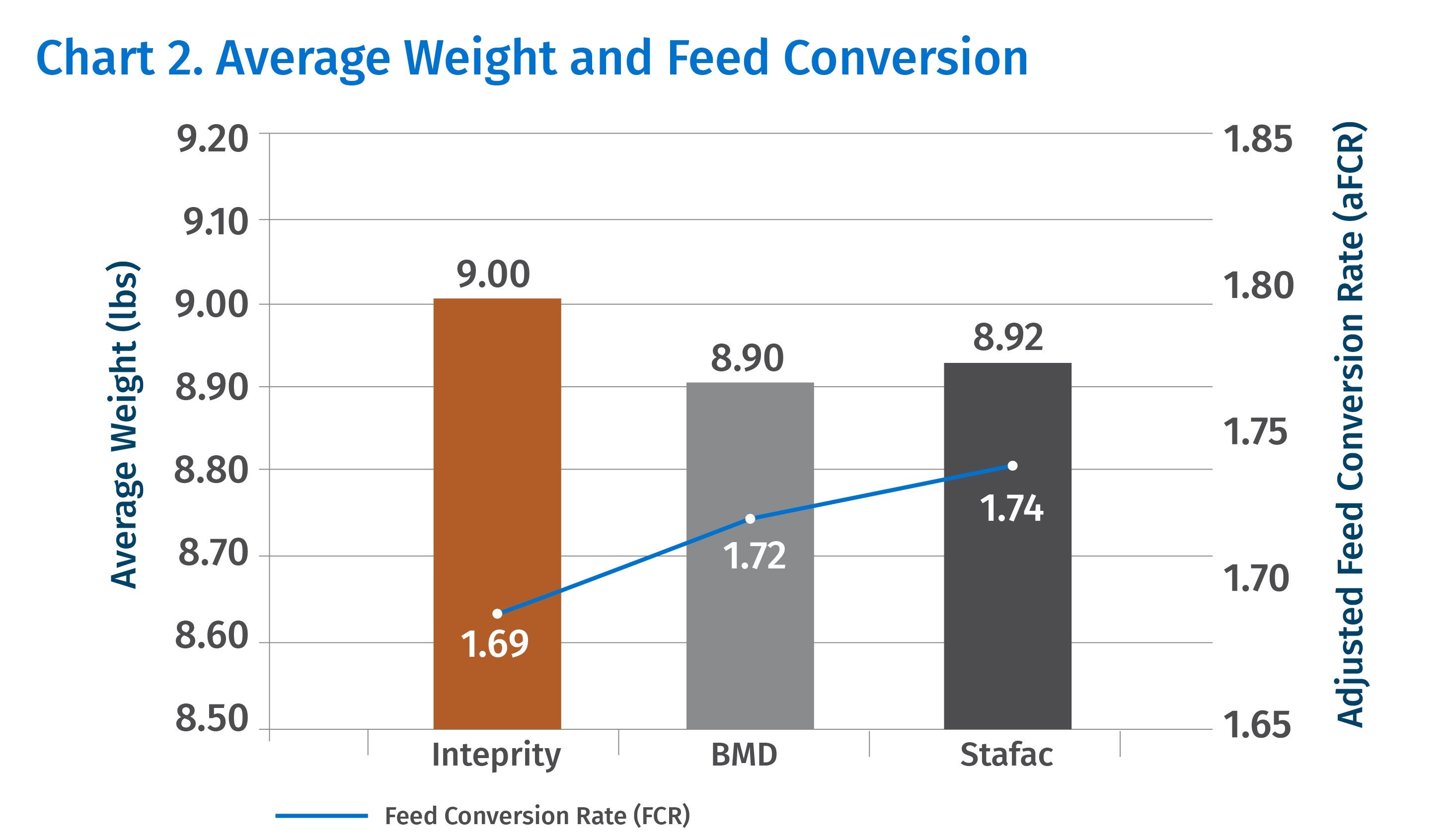
Additionally, using Inteprity at 22.7 g/ton in the starter feed on days 0-21 (Chart 2) improved weight gain and adjusted feed conversion compared to feeding BMD or Stafac during the same period.
Learn More About Inteprity
Get more information on how Inteprity can help keep your poultry flock healthy.
Inteprity Product Label
Important use and safety information for Inteprity (Avilamycin type A medicated article).
Inteprity Safety Data Sheet
Information including guidelines for the safe use, handling and storage of Inteprity.
Fight Necrotic Enteritis with Inteprity

Contact your Elanco representative to learn how Inteprity can fit into your operation. To locate the closest representative or get more information about Elanco poultry products, contact us.
Usage Information
The label contains complete use information, including cautions and warnings. Always read, understand and follow the label and use directions.
Inteprity directions for use:
For the prevention of mortality caused by necrotic enteritis associated with Clostridium perfringens in broiler chickens:
- Avilamycin is to be fed at 13.6 to 40.9 grams per ton of Type C medicated feed (15 to 45 ppm) as the sole ration for 21 consecutive days
- Feed to chickens that are at risk of developing, but not yet showing clinical signs of, necrotic enteritis associated with Clostridium perfringens
Inteprity Important Safety Information
CAUTION: Federal law restricts medicated feed containing this veterinary feed directive (VFD) drug to use by or on the order of a licensed veterinarian. To assure responsible antimicrobial drug use in broiler chickens, treatment administration must begin on or before 18 days of age. When using in combination with Maxiban, Monteban, Coban and Bio-Cox, treatment and administration must begin on or before 18 days of age.
The safety of avilamycin has not been established in chickens intended for breeding purposes.
Avilamycin has not been demonstrated to be effective in broiler chickens showing clinical signs of necrotic enteritis prior to the start of medication.
The Veterinary Feed Directive (VFD) expiration date must not exceed 90 days from the date of issuance. VFDs for avilamycin shall not be refilled.
Coban directions for use:
As an aid in the prevention of coccidiosis caused by Elmeria necatrix, E. mivati and E. maxima in broiler chickens:
- Feed Coban at 90-110 g/ton
- Feed continuously as the sole ration
- Requires a zero-day withdrawal (when fed according to the label)
For the prevention of coccidiosis in turkeys caused by Eimeria adenoeides, E. meleagrimitis and E. gallopavonis:
- Feed Coban at 54-90 g/ton to turkeys
- Feed continuously as the sole ration
- Requires a zero-day withdrawal (when fed according to the label)
Important Safety Information
WARNING: Do not feed to laying chickens. Do not feed to chickens over 16 weeks of age.
CAUTION: Do not allow horses, other equines, mature turkeys, or guinea fowl access to feed containing monensin. Ingestion of monensin by horses and guinea fowl has been fatal. Some strains of turkey coccidia may be monensin tolerant or resistant. Monensin may interfere with development of immunity to turkey coccidiosis. In the absence of coccidiosis in broiler chickens the use of monensin with no withdrawal period may limit feed intake resulting in reduced weight gain. Not for broiler breeder replacement chickens.
Maxiban directions for use:
For the prevention of coccidiosis in broiler chickens caused by Eimeria necatrix, E. tenella, E. acervulina, E. brunetti, E. mivati and E. maxima:
- Feed Maxiban at 54-90 g/ton
- Feed continuously as the sole ration
- Requires a zero-day withdrawal (when fed according to the label), some combination use requires 5-day withdrawal
CAUTION: Nicarbazin medicated broilers may show reduced heat tolerance if exposed to high temperature and high humidity. Provide adequate drinking water and ventilation. Do not allow adult turkeys, horses or other equines access to formulations containing narasin. Ingestion of narasin by these species has been fatal. Do not feed to laying hens.
Monteban directions for use:
For the prevention of coccidiosis caused by Eimeria necatrix, E. tenella, E. acervulina, E. brunetti, E. mivati and E. maxima in broiler chickens:
- Feed Monteban at: 54–90 g/ton
- Feed continuously as the sole ration
- Requires a zero-day withdrawal (when fed according to the label)
CAUTION: Do not allow adult turkeys, horses or other equines access to narasin formulations. Ingestion of narasin by these species has been fatal.
Coban, Inteprity, Maxiban, and Monteban are trademarks of Elanco or its affiliates. Other product names are trademarks of their respective owners.
